Welcome to ED Medical Equipment Engineering Co., Ltd.
- About Us
-
- Company Profile
-
- Honor
-
- Growing experience
-
- The enterprise culture
-
- Equity structure
-
- Join Aide
-
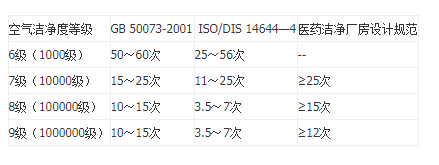
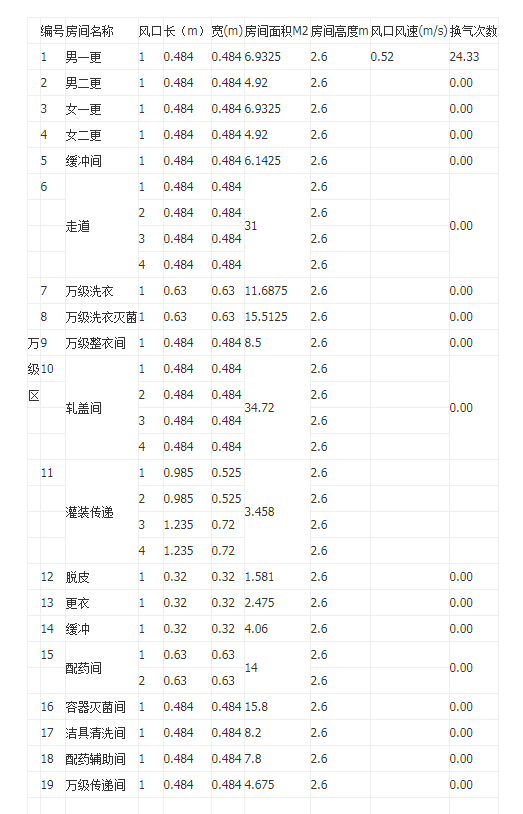
- The wind calculation show
-
- Service support
-
- Sales network
-
- Organizational structure
- News
-
- Company news
-
- Industry Information
-
- conceptual design
-
- Equipment maintenance
-
- technical service
-
- Data Download
-
- Cleaning principle
-
- Common problem
-
- Purification classification
-
- construction technology
-
- Purification drawings
-
- Supply information
- Products
-
- Purification engineering installation
-
- Purification equipment
-
- hepa box
-
- Supporting products of ED
-
- Laboratory support
-
- Medical Writing Table
-
- Control panel
-
- Stainless steel instrument cabinet
-
- Operating Room Air Source Box
-
- Medical viewing lamp
-
- return air grille
-
- Operating room socket box
-
- Medical Hand Washing Pool
-
- Transfer window
-
- Air shower
-
- Air filter
-
- Laminar air supply smallpox
-
- Clean lamp
-
-
- Medical Purification Door
-
-
- Suspension bridge crane series
-
- Bed and Ward Care Series
-
- Clean room air conditioning system
-
- Surgery table series
-
- Shadowless Lamp Series
- Cases
-
- Workshop purification engineering
-
- Hospital decontamination
-
- Laboratory decontamination
-
- National cleaning customer case
-
- Mobile Operating Vehicle Purification Project
-
- Air purification project
- Human Resources
-
- Human Resources
-
- Recruitment information
- Contact Us
-
- Contact Us
-
- Message
PC Edition Touch Edition Statistics Manager